Outbreaks of other species include native species (as opposed to introduced species; Section 3.6.3) that can experience a rapid increase in abundance, biomass or population. Outbreaks of the naturally occurring crown-of-thorns starfish and coral disease are described in Sections 3.6.1 and 3.6.2, respectively. Outbreaks and blooms are often a sign of ecosystem stress and can be harmful or lethal to other marine species. Some outbreaks can also pose a threat to human health, either through direct consumption of ciguatoxins in fish, inhalation of microscopic organisms causing the outbreak,1043 or exposure to toxic cyanobacteria in water.1044

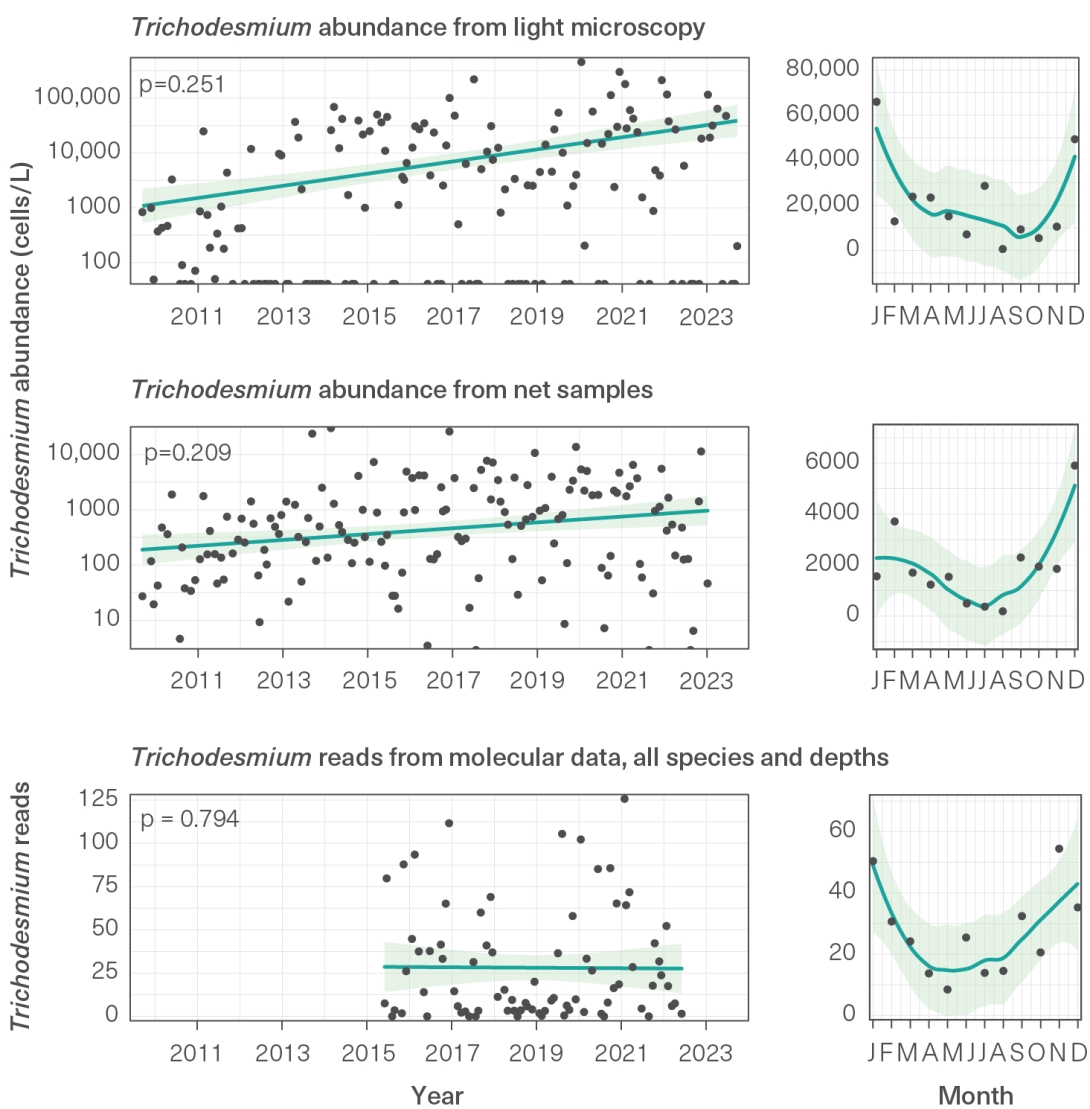
Trichodesmium is a pelagic cyanobacterium that can create slicks when significant numbers of cells accumulate and float to the water surface. On exposure to sunlight, the cells die, leaving floating pungent slicks that look like sawdust on the surface of the water. Slicks are more common under hot, calm conditions in spring and summer, aided by slow, circulating surface currents.1045 Phosphorus and iron may also influence Trichodesmium growth and bloom formation.724,1046 Two species of Trichodesmium are present within the Region, and T. thiebautii is reported as the dominant species at the Integrated Marine Observing System (IMOS) Yongala National Reference Station near Ayr.251 Data show a long-term increase in Trichodesmium abundance has occurred and that seasonally lower abundances are observed during winter periods 358 (Figure 3.16). Modelling and remote sensing analyses suggest that the largest Trichodesmium blooms arise in the south of the Region,1047 and their peak abundance occurs at a water temperature of 26 degrees Celsius, usually in spring.724 Increased nitrogen fixation rates have been associated with elevated phosphorus conditions 724 and reduced dissolved nitrogen.804
Figure 3.16
Trichodesmium abundance, Yongala Integrated Marine Observing System (IMOS) National Reference Station, 2010 to 2023
Indices of Trichodesmium abundance at the Yongala IMOS National Reference Station, from phytoplankton bottle samples using light microscopy (top), zooplankton net samples (middle) and molecular analysis of bottle samples (bottom). Green shading shows 95 per cent confidence interval, p-values indicate whether trend is statistically significant (p < 0.05). Left graphs use a linear model, right graphs use a loess smoother. Source: Richardson et al. (2023)251 using data from Australia’s IMOS, enabled by the National Collaborative Research Infrastructure Strategy (NCRIS) and operated by a consortium of institutions as an unincorporated joint venture with the University of Tasmania as Lead Agent
Drupella are corallivorous (coral-eating) marine snails that occur naturally within the Region, commonly in aggregations.1048 In high numbers, Drupella can cause damage and mortality in corals through direct predation 1049 and by facilitating the spread of disease.1050,1051 Overfishing of predators, elevated nutrients and warming sea temperatures may exacerbate outbreaks.1052,1053 Counts of Drupella snails and feeding scars are provided by a number of monitoring programs within the Region.1054 Since 2019 high densities of Drupella have been recorded at some reefs, in most cases at densities below the threshold where detrimental impact on coral communities is expected,185 and no significant outbreaks are known to have occurred.
More than 100 species of jellyfishes occur within the Region, most of which are considered harmless.1055 The most well-known species are those that are dangerous to humans, including the Australian box jellyfish and Irukandji. Jellyfish movements and behaviour are influenced by environmental factors.1056 Australian box jellyfish are largely distributed close to mainland shores.1055,1057 Since 2019, modelling suggests that Irukandji presence and abundance near Cairns is highly variable and cannot be explained by environmental predictors, such as wind or rainfall.1058 Survey approaches using eDNA 1059 and unmanned aerial vehicles 1060 are providing new ways to improve detection of certain life stages and species of jellyfish. While jellyfish blooms are well known globally,1061 large aggregations of box jellyfish species are relatively uncommon 1058 and no information on trends is available within the Region.
A long-term increase in Trichodesmium abundance has continued at the Yongala National Reference Station. High numbers of Drupella snails have been recorded at some reefs, but no outbreaks have been reported.


