Primary production, which refers to the initial synthesis of organic compounds from carbon dioxide and energy, provides the basis for all biological systems. Nearly all primary production within the Region occurs through the process of photosynthesis. Primary producers within the Region include phytoplankton, benthic algae, mangroves, and seagrasses. Many reef organisms, notably including corals, benefit from symbiotic associations with primary producers (Section 3.4.6). Algal symbionts of corals transfer between 40 and 80 per cent of their photosynthetically fixed carbon to their hosts, supporting coral growth and metabolism 800 and contributing to their evolutionary success.801
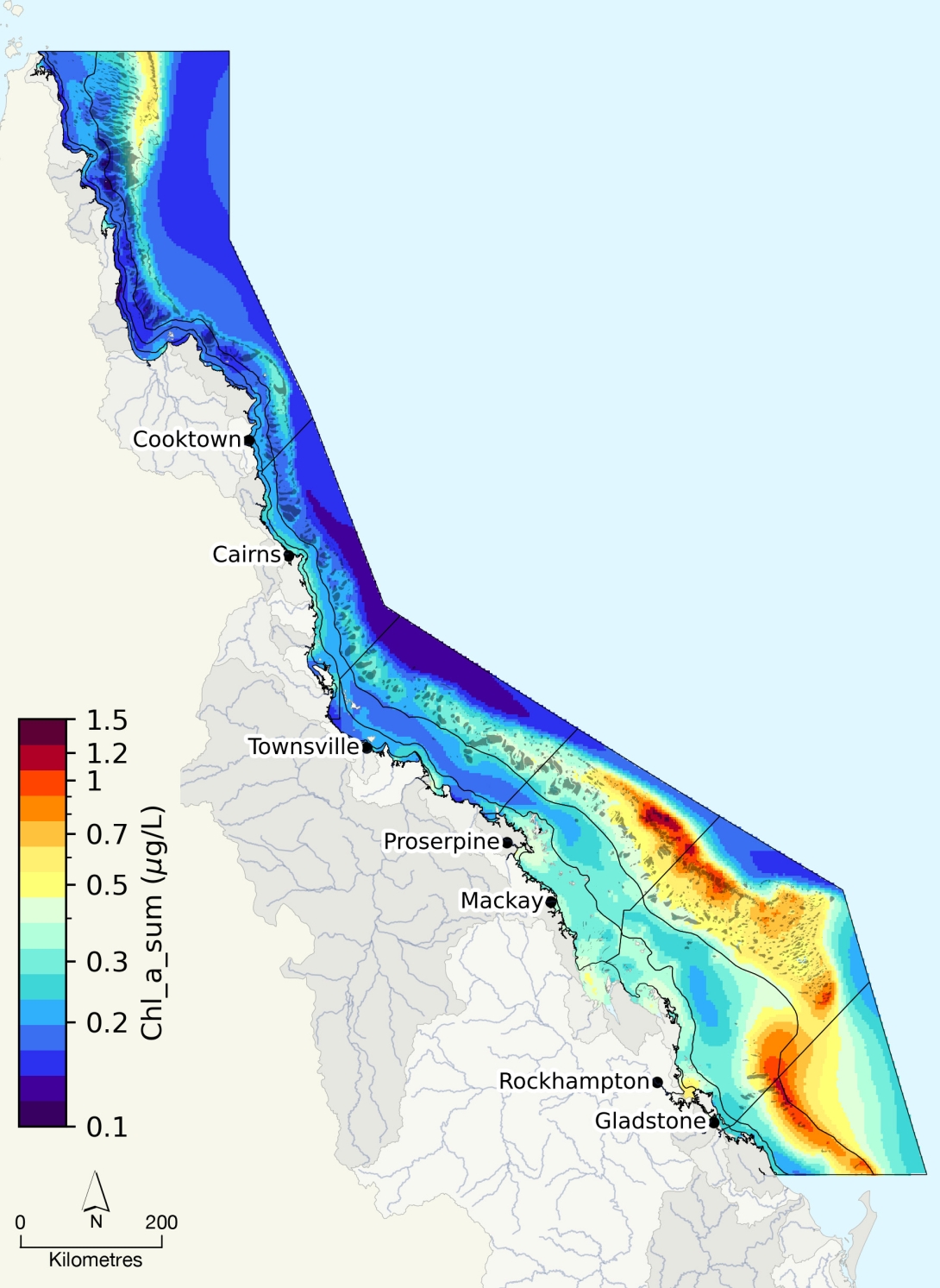
Photosynthesis is closely linked to the availability of light (Section 3.2.7) and generally occurs in the upper water column at depths of less than 100 metres.672 Rates of primary production depend on available light and the efficiency with which organisms can use it for photosynthesis.682 The availability of inorganic nutrients, such as nitrogen and phosphorus, is also critical in determining rates of primary production. Within the Region, peaks in primary productivity tend to occur in inshore waters as a result of elevated fluxes of nutrients from river flows and tidal mixing 802 particularly during the wet season.803 Nutrients are also supplied through inflow from the Coral Sea, upwelling and resuspension of sediments,802 and via nitrogen-fixing bacterioplankton.804 In some cases, increased primary productivity can signal a decline in water quality,363 or it may indicate phase shifts and the presence of prior ecological disturbances.749