Herbivory involves the removal and consumption of plant matter. It is a key ecological process for a functional coral reef ecosystem,805 providing nutrition for the consumer and influencing the location, health and composition of plant communities.441,806 Marine herbivory includes removal of plant matter by large marine herbivores (such as green turtles and dugongs142); cropping, grazing and excavation of benthic algae by fishes 807 and invertebrates; 772 and the consumption of phytoplankton by micro-grazers, such as amphipods and isopods.808 Herbivory is also observed in early life stages of the coral‑eating crown-of-thorns starfish.349,809,810
Herbivory plays a critical role in the recovery of coral reefs after disturbance
The dynamic balance between rates of herbivory (algal removal) and primary production (algal growth) is central to the functioning of coral reef ecosystems.811 Herbivorous fish and other species predominantly target algal turfs and microbes living within reef structures (endolithic algae and microbes),812 thereby preventing macroalgae from becoming established.813 In this way, herbivory plays a critical role in the recovery of coral reef communities after disturbance.814 Grazing of macroalgae on coral reefs is also performed predominantly by herbivorous fishes 807,811 and to a lesser extent by molluscs, echinoderms, and crustaceans,772 but this grazing is not closely linked to macroalgae biomass.815 In seagrass meadows, grazing by dugongs and green turtles is important for habitat structure and condition 142 and can aid dispersal and germination of seagrass species (Sections 2.3.4 and 8.3.5).143,808 Environmental disturbances, such as cyclones and coral bleaching, can benefit herbivores by increasing available algal substrate, but can result in herbivore assemblages with reduced richness that are potentially more vulnerable to chronic localised stresses.816,817
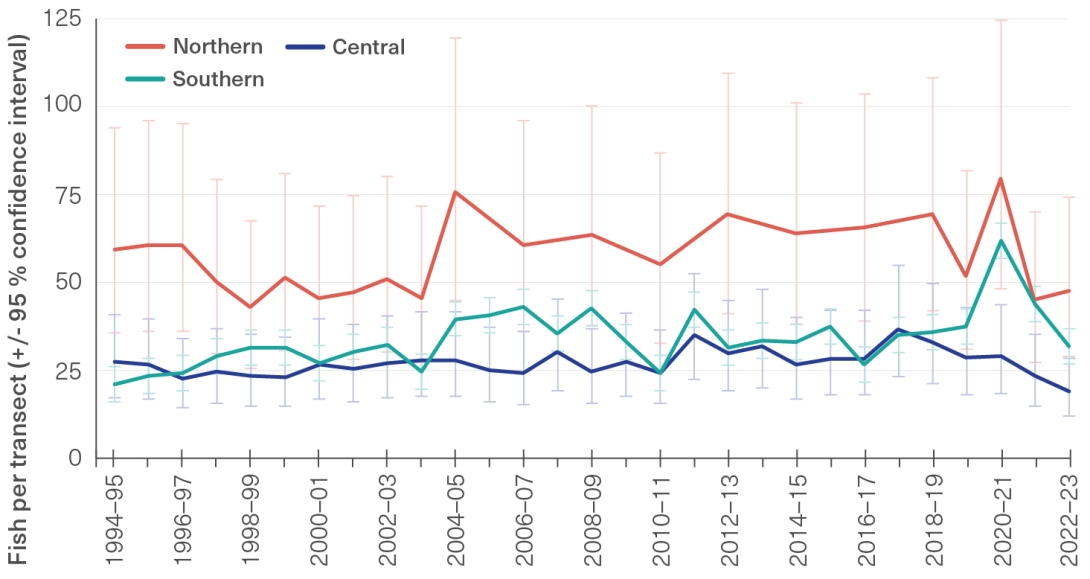
Although novel survey methods are providing insights into ecosystem-level patterns of herbivory,818,819 the process of herbivory is not routinely monitored. As a readily available simple metric, herbivore abundance may give some insight into relative herbivory rates, however the changing area of algal substrate (linked to coral cover) is also a key determinant of the intensity and effectiveness of herbivory on reefs.820 Herbivory is seen across the Region, from inshore habitats to the depths on the outer-shelf reefs where light penetration allows for photosynthesis and therefore algal growth.197 Surveyed abundance of herbivorous fishes (such as parrotfish, surgeonfish and rabbitfish) has fluctuated since 2019, although not beyond the normal range of long-term natural variability (Figure 3.13).386 Documented declines in dugongs 821 (Section 2.4.16) and northern populations of green turtles (Section 2.4.10) may lead to reduced herbivory in the Region.
Despite its central importance to ecosystem functioning, an in-depth understanding of the process of herbivory across the Region and the mechanisms that affect it remains elusive.811 This process is undertaken by a wide range of species at varying scales and with diverse effects on ecosystem function across different habitats. Overall, herbivory has likely remained stable since 2019. Declines in some populations of dugongs, green turtles and potentially in herbivorous fishes may lead to reduced levels of herbivory in certain areas.
The balance between herbivory and primary production is a key to reef health
Figure 3.13
Regional abundance of herbivorous fishes, 1994–95 to 2022–23
Estimated mean abundance (with 95 per cent confidence intervals) of herbivorous fishes (including parrotfish, surgeonfish and rabbitfish) per transect across northern, central and southern regions of the Reef from 1994–95 to 2022–23 as part of Australian Institute of Marine Science (AIMS) Long-term Monitoring Program surveys. Fish are counted along 50-metre by 5-metre transects, and 15 transects are completed per reef surveyed.822 The average number of reefs visited in a year was 50. The maximum visited was 69, in 2021–22. Fish estimated to be less than 1 year old (distinguished by size and colouration) are excluded from counts to reduce variability. Source: AIMS Long-term Monitoring Program.386