363.
Moran, D., Robson, B., Gruber, R., Waterhouse, J., Logan, M., et al. 2023, Marine Monitoring Program Annual Report 2021-22 Water Quality, Great Barrier Reef Marine Park Authority, Townsville.
579.
Gruber, R., Moran, D., Robson, B., Waterhouse, J., Logan, M., et al. 2024, Marine Monitoring Program: Annual report for inshore water quality monitoring 2022–23. Report for the Great Barrier Reef Marine Park Authority, Great Barrier Reef Marine Park Authority, Townsville.
586.
Lewis, S., Bainbridge, Z. and Smithers, S. 2024, 2022 Scientific Consensus Statement: Summary | Evidence Statement for Question 3.1: What are the spatial and temporal distributions of terrigenous sediments and associated indicators within the GBR? in 2022 Scientific Consensus Statement on land-based impacts on Great Barrier Reef water quality and ecosystem condition, eds J. Waterhouse, M. Pineda and K. Sambrook, Commonwealth of Australia and Queensland Government.
597.
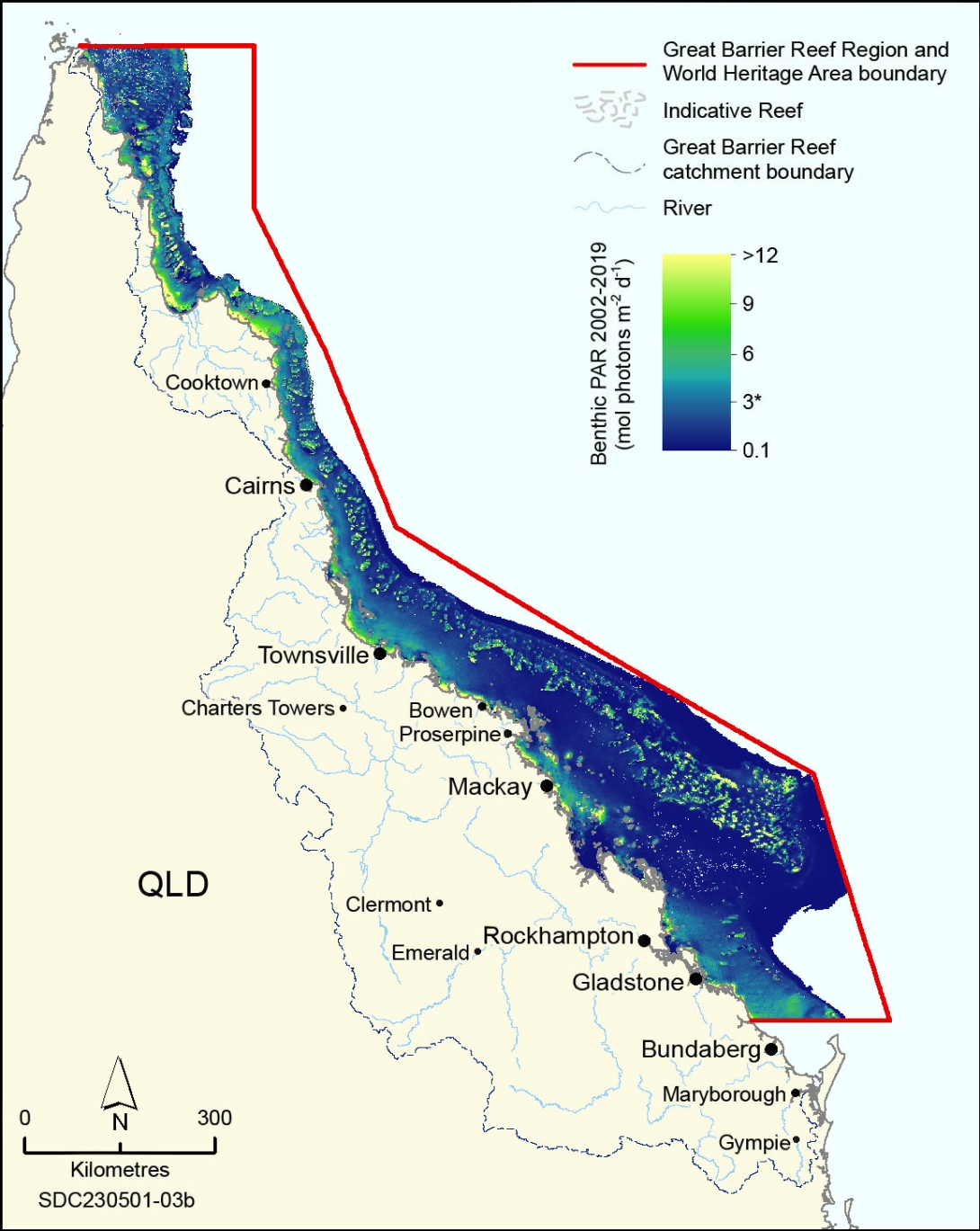
Canto, M.M., Fabricius, K.E., Logan, M., Lewis, S., McKinna, L.I.W., et al. 2021, A benthic light index of water quality in the Great Barrier Reef, Australia, Marine Pollution Bulletin 169: 112539.
672.
Hoegh-Guldberg, O. and Dove, S. 2019, Primary production, nutrient recycling and energy flow through coral reef ecosystems, The Great Barrier Reef: Biology, Environment and Management. Second Edition: 85-100.
673.
Strahl, J., Rocker, M.M. and Fabricius, K.E. 2019, Contrasting responses of the coral Acropora tenuis to moderate and strong light limitation in coastal waters, Marine Environmental Research 147: 80-89.
674.
Hoegh-Guldberg, O. and Dove, S. 2008, Primary production, nutrient recycling and energy flow through coral reef ecosystems, in The Great Barrier Reef: Biology, Environment and Management, eds P. Hutchings, M. Kingsford and O. Heogh-Guldberg, CSIRO Publishing, Collingwood, pp. 59-73.
675.
Muir, P.R., Pichon, M., Squire, L. and Wallace, C.C. 2019, Acropora tenella, a zooxanthellate coral extending to 110-m depth in the northern Coral Sea, Marine Biodiversity 49: 809-814.
676.
Englebert, N., Bongaerts, P., Muir, P., Hay, K.B. and Hoegh-Guldberg, O. 2015, Deepest zooxanthellate corals of the Great Barrier Reef and Coral Sea, Marine Biodiversity 45: 1-2.
677.
Jones, R., Pineda, M., Luter, H.M., Fisher, R., Francis, D., et al. 2021, Underwater Light Characteristics of Turbid Coral Reefs of the Inner Central Great Barrier Reef, Frontiers in Marine Science 8: 727206.
678.
Bessell-Browne, P., Negri, A.P., Fisher, R., Clode, P.L. and Jones, R. 2017, Impacts of light limitation on corals and crustose coralline algae, Scientific Reports 7(1): 11553.
679.
Luter, H.M., Pineda, M., Ricardo, G., Francis, D.S., Fisher, R., et al. 2021, Assessing the risk of light reduction from natural sediment resuspension events and dredging activities in an inshore turbid reef environment, Marine Pollution Bulletin 170: 112536.
680.
Noonan, S.H., DiPerna, S., Hoogenboom, M.O. and Fabricius, K.E. 2022, Effects of variable daily light integrals and elevated CO 2 on the adult and juvenile performance of two Acropora corals, Marine Biology 169: 1-15.
681.
Brunner, C.A., Ricardo, G.F., Uthicke, S., Negri, A.P. and Hoogenboom, M.O. 2022, Effects of climate change and light limitation on coral recruits, Marine Ecology Progress Series 690: 65-82.
682.
Hochberg, E.J., Pisapia, C., Carpenter, R.C. and Hall, S. 2024, Light-use efficiency for coral reef communities and benthic functional types, Limnology and Oceanography 69(3): 712-722.
683.
Gonzalez‐Espinosa, P.C. and Donner, S.D. 2021, Cloudiness reduces the bleaching response of coral reefs exposed to heat stress, Global Change Biology 27(15): 3474-3486.
684.
Rosic, N., Rémond, C. and Mello-Athayde, M.A. 2020, Differential impact of heat stress on reef-building corals under different light conditions, Marine Environmental Research 158: 104947.
685.
Lohr, K.E., Camp, E.F., Kuzhiumparambil, U., Lutz, A., Leggat, W., et al. 2019, Resolving coral photoacclimation dynamics through coupled photophysiological and metabolomic profiling, Journal of Experimental Biology 222(8): jeb195982.
686.
DiPerna, S., Hoogenboom, M., Noonan, S. and Fabricius, K. 2018, Effects of variability in daily light integrals on the photophysiology of the corals Pachyseris speciosa and Acropora millepora, PLoS One 13(9): e0203882.
687.
Carter, A.B., Collier, C., Rasheed, M.A., McKenzie, L. and Udy, J. 2018, A framework for defining seagrass habitat for the Great Barrier Reef, Australia, Reef and Rainforest Research Centre.
688.
Carter, A.B., Collier, C., Lawrence, E., Rasheed, M.A., Robson, B.J., et al. 2021, A spatial analysis of seagrass habitat and community diversity in the Great Barrier Reef world heritage area, Scientific Reports 11(1): 22344.
689.
Collier, C., Chartrand, K., Honchin, C., Fletcher, A. and Rasheed, M. 2016, Light Thresholds for Seagrasses of the GBRWHA: a synthesis and guiding document: including knowledge gaps and future priorities. report to the National Environmental Science Program, Reef and Rainforest Research Centre Limited, Cairns.
690.
Collier, C.J., Waycott, M. and McKenzie, L.J. 2012, Light thresholds derived from seagrass loss in the coastal zone of the northern Great Barrier Reef, Australia, Ecological Indicators 23: 211-219.
691.
McMahon, K., Collier, C. and Lavery, P.S. 2013, Identifying robust bioindicators of light stress in seagrasses: a meta-analysis, Ecological Indicators 30: 7-15.
692.
Chartrand, K.M., Szabó, M., Sinutok, S., Rasheed, M.A. and Ralph, P.J. 2018, Living at the margins–The response of deep-water seagrasses to light and temperature renders them susceptible to acute impacts, Marine Environmental Research 136: 126-138.
693.
Waterhouse, J., Brodie, J., Tracey, D., Smith, R., Vandergragt, M., et al. 2017, Scientific Consensus Statement 2017: A synthesis of the science of land-based water quality impacts on the Great Barrier Reef, Chapter 3: The risk from anthropogenic pollutants to Great Barrier Reef coastal and marine ecosystems, State of Queensland, Brisbane.
694.
Magno-Canto, M.M., McKinna, L.I., Robson, B.J. and Fabricius, K.E. 2019, Model for deriving benthic irradiance in the Great Barrier Reef from MODIS satellite imagery, Optics Express 27(20): A1350-A1371.
695.
Great Barrier Reef Foundation, Bureau of Meteorology, CSIRO, Australian Institute of Marine Science and Queensland Government, 2019, eReefs, Australian Government, <https://ereefs.org.au/ereefs>.